Abstract
BACKGROUND:
Hematopoietic stem cell transplantation (HSCT) is the treatment option for several malignant and non-malignant diseases. In HSCT, T-cell mediated immunity is kept low to prevent graft versus host disease (GvHD), making the patient susceptible to viral infections. The most common viral infections after HSCT includes Cytomegalovirus (CMV), Epstein-Barr virus (EBV), BK virus (BKV), adenovirus (ADV), and human herpesvirus 6 (HHV-6). Pharmacological options are available to treat these infections but have limitations due to drug resistance, toxicities, and do not confer long-term protection. The best innovative way to overcome the limitations of antiviral and treat the condition is adoptive immunotherapy with viral-specific T (VST) cells. The present systematic review and meta-analysis aimed to evaluate studies exclusively about the dose of viral-specific T cells, infusion time, efficacy in treating infection, infection relapse, safety, and infusion-related side effects.
METHODS:
Following the preferred reporting items for systemic reviews and Meta-analysis (PRISMA) guidelines, 562 articles from 3 databases (PubMed, Cochrane, and Clinicaltrials.gov) using MeSH terms and keywords for "Adenoviridae" "Cytomegalovirus," "BK Virus" AND "Hematopoietic Stem Cell Transplantation" AND "Virus-specific T cells" from the date of inception to Jan 16, 2022 were reviewed. Studies were screened by title, abstract, and full text. The original studies reporting adult and pediatric HSCT patients who underwent VST cell therapy for CMV/BK/ADV viral infection were included. At the same time, reviews, duplicate, and non-relevant articles were excluded. A total of nine studies (clinical trials) were included. Pooled analysis was done using the 'meta-package (R Studio software), and a random-effects model was used to estimate the pooled prevalence with 95 % confidence intervals (CI).
RESULTS:
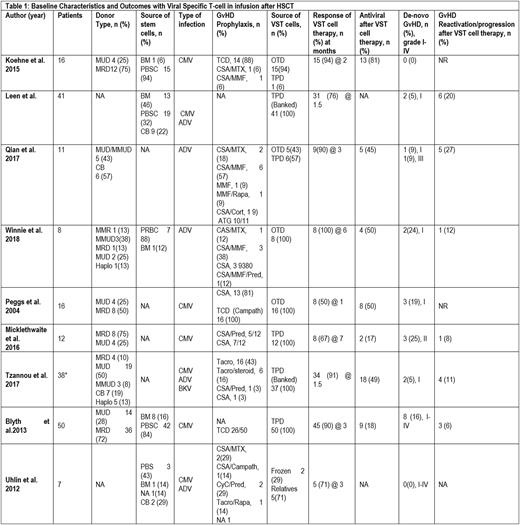
We identified 199 participants in the 9 prospective studies with allo-HSCT, who received VST cell therapy. (Table1) Median age of the patients was 31.8 (0.5-68) years. The median duration of T-cell therapy after HSCT was 62.5 (4-164) days and median follow up time after giving T-cell therapy was 7.16 (1.38-80) months, provided by five included studies. The type of donor was matched unrelated donor (MUD) 34.4% (52/151), matched related donor (MRD) 40% (61/151), mismatched related donor (MMR) 0.66% (1/151), mismatched unrelated donor (MMUD) 1.9% (3/151), and haploidentical 3.9% (6/151) as reported by seven studies. The source of stem cells was bone marrow (BM) in 19.5% (24/122), peripheral blood stem cell (PBSC) in 70.5% (86/122), and cord blood (CB) in 9% (11/122) as reported by five studies. The pooled clearance of viral activation with VST was 82.8% (95% CI 0.72-0.91, I2=58%, n=199) at a median of 1.77 (1.5-3) months, while the pooled clearance of viral activation with antiviral therapy after VST was estimated as 42.9% (95% CI 0.25-0.61, I2=78%, n=151), according to data by seven studies. The pooled incidence of de-novo GvHD was 9.1% (95% CI 0.036-0.16, I2=40%, n=199) and pooled incidence of GvHD reactivation/progression after VST cell therapy was 9.9% (95% CI 0.05-0.15, I2=0%, n=160) based on data provided by six studies.
CONCLUSION:
Infusion of virus specific T cells in CMV, Adenovirus, and BK virus post hematopoietic stem cell transplant has shown excellent outcomes and survival with favourable safety profile. VST cells infusion represents a good choice to treat viral reactivation/relapse occurring after HSCT.
Disclosures
Abhyankar:Incyte: Consultancy, Research Funding, Speakers Bureau; Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk:BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; CRISPR Therapeutics: Consultancy; Sana: Honoraria; In8bio, Inc.: Other: IIT Clinical Trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal